April L Risinger, Ph.D.
Associate Professor and Greehey Distinguished Chair in Targeted Molecular Therapeutics
Currently seeking Ph.D. students
The research in the Risinger Laboratory is in the area of cancer pharmacology with a focus on microtubule targeted agents and other natural products for the treatment of drug resistant solid tumors.
There are three overarching goals of our research, including the identification of biomarkers that can guide a more rational choice among clinically approved microtubule targeted agents for the treatment of women with breast cancer or gynecological malignancies, the development of novel classes of microtubule targeted agents that retain efficacy in drug-resistant disease, and identifying strategies to alleviate the toxicities associated with the use of microtubule-targeted agents and other chemotherapeutics.
Drug Discovery and Development
Each of the unique classes of microtubule-targeted chemotherapeutics currently in clinical use or preclinical evaluation was originally identified as a natural product. Therefore, we collaborate closely with natural product chemists to identify and characterize novel natural compounds for their potential as cancer therapeutics as well as with synthetic medicinal chemists to optimize their potential for translation into the clinic. This includes our long-standing work on the identification and optimization of covalent microtubule targeted agents, including the plant-derived taccalonolides. My laboratory found that the taccalonolides bind covalently to a distinct site on tubulin from other stabilizers with a high degree of specificity that allows them to retain efficacy in clinically relevant drug resistant models both in vitro and in vivo. I am an inventor on two issued patents on this class of microtubule stabilizers and we have active projects in the laboratory focused on optimizing their pharmacokinetic properties and improving tumor targeting. We are also actively working on the optimization of other covalent microtubule targeted natural products, including pironetin and zampanolide, for the treatment of drug resistant human cancers.
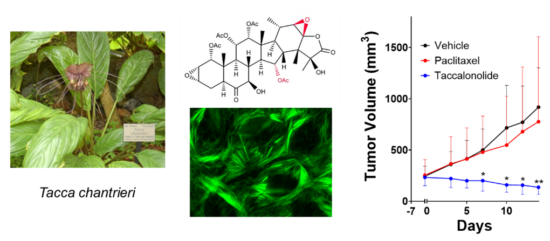
Improving the Targeted use of Approved Chemotherapeutics
In addition to identifying new therapeutics, my laboratory is also actively investigating the underappreciated mechanistic differences between microtubule targeted agents currently approved for the treatment of cancer to guide their more rational and targeted use in the clinic. We have identified that the microtubule destabilizer eribulin is unique from the microtubule stabilizing taxanes in its ability to acutely activate the cGAS-STING pathway independent of its antimitotic effects through a mechanism involving mitochondrial DNA release. We further found that eribulin is highly synergistic with STING agonists in clinical development and able to promote improved antitumor immunity and tumor regression in vivo. We are also uncovering differences between the effects of these clinically approved drugs on cytoskeletal mediators implicated in oncogenesis. These preclinical findings have led to collaborations with our Mays Cancer Center to test the hypothesis that our findings can be used to identify molecular biomarkers to support the rational use of these drugs in combination with immunotherapeutics as well as guide the choice between microtubule stabilizing and destabilizing chemotherapeutics in the treatment of breast cancer patients where both classes of agents are approved and currently used with no molecular guidance.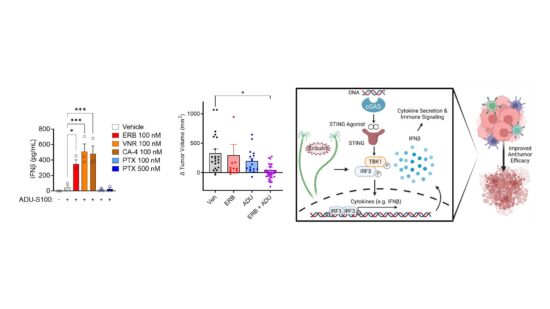
Improving treatment by alleviating toxic side effects
Some of the major hurdles to effective cancer treatment are the toxic side effects associated with microtubule targeted agents and other chemotherapeutics. I am actively collaborating with neuroscientists and pain researchers to characterize and alleviate the neuropathic and cognitive deficits associated with cancer therapy. These collaborations have led to the development of models that allow monitoring of both the neurological and antitumor activities of chemotherapeutics in the same animal so that we can identify interventions that mitigate the deleterious side effects of these drugs without compromising their anticancer efficacy.
-
Professional Background
Education
- 2014 - Postdoctoral Training - Pharmacology - The University of Texas Health Science Center at San Antonio
- 2007 - PhD - Cell Biology - Massachusetts Institute of Technology (MIT)
- 2000 - BS - Biochemistry - Texas A&M University
Highlights
Select Honors and Awards
Best Undergraduate Thesis in Molecular Genetics, Texas A&M University
Barbara Bowman Postdoctoral Award and Fellowship
Voelcker Young Investigator Award
Jack L. Beal Award for best paper by an earlier career investigator in the
Journal of Natural ProductsGreehey Distinguished Chair in Targeted Molecular Therapeutics
Appointments
- 09/2022 - Associate Professor - The University of Texas Health Science Center at San Antonio, Pharmacology, San Antonio
- 1/2014 - Assistant Professor - The University of Texas Health Science Center at San Antonio, Pharmacology, San Antonio
-
- Research & Grants
- Publications